PDR / DMO
Important safety information for patients treated with Ongavia (ranibizumab) with proliferative diabetic retinopathy (PDR) and/or visual impairment due to diabetic macular oedema (DMO)
▼ This medicine is subject to additional monitoring. This will allow quick identification of new safety information. You can help by reporting any side effects you may get.
Suspected adverse reactions should be reported to the MHRA via Yellow Card Scheme at www.mhra.gov.uk/yellowcard.
Your guide to therapy with Ongavia in the treatment of
-
Proliferative diabetic retinopathy (PDR)
-
Diabetic Macular Oedema (DMO)
Please refer additionally to the Patient Information Leaflet to read what Ongavia is and what it is used for. The Patient Information Leaflet can be read or listened to on the Ongavia website.
Summary of important information
Contact your doctor as soon as possible if you experience any of the following symptoms after treatment with Ongavia:
- Pain
- Light sensitivity/ tearing
- Swollen lids or other swelling
- Light flashes
- Seeing flies, black spots or coloured halos
- Drying of the surface of your eye
- Increasing redness
- Blurred, distorted or sudden loss of vision
What is Ongavia?
In diseases such as PDR and DMO, abnormal blood vessels grow in the eye. These can leak and cause vision loss.
Ongavia contains ranibizumab as the active medicinal ingredient. Ranibizumab specifically recognises and blocks the action of new blood vessel growth in the eye, and in turn can help to stop leakage and vision loss.
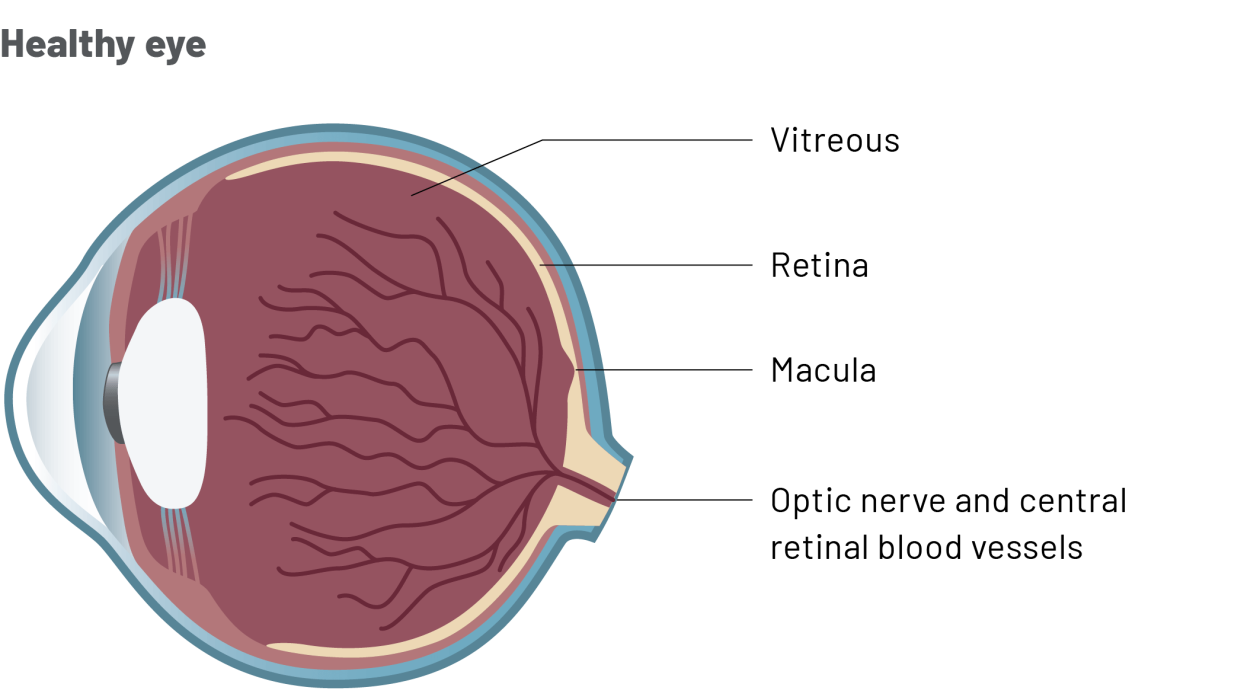
Why have I been prescribed Ongavia?
You have been diagnosed with DMO or PDR.
DMO
- DMO is a condition that affects the macula, a part of the retina at the back of the eye.
- The macula is the area that lets you see sharply in the centre of your vision.
- In patients with diabetes, having high blood sugar for a long time may cause damage to blood vessels in the eye. Damaged blood vessels leak into the retina and the accumulation of this fluid causes damage to the retina and vision loss.
- In some cases, abnormal new blood vessels also start to grow under the macula, which may contribute to your loss of vision.
PDR
- Diabetic retinopathy (DR) is a common eye condition caused by diabetes.
- It is classified as either non-proliferative DR (early-stage disease) or proliferative DR (end stage disease in which abnormal new blood vessels grow in the retina).
- PDR can severely threaten vision, particularly in the later stages.
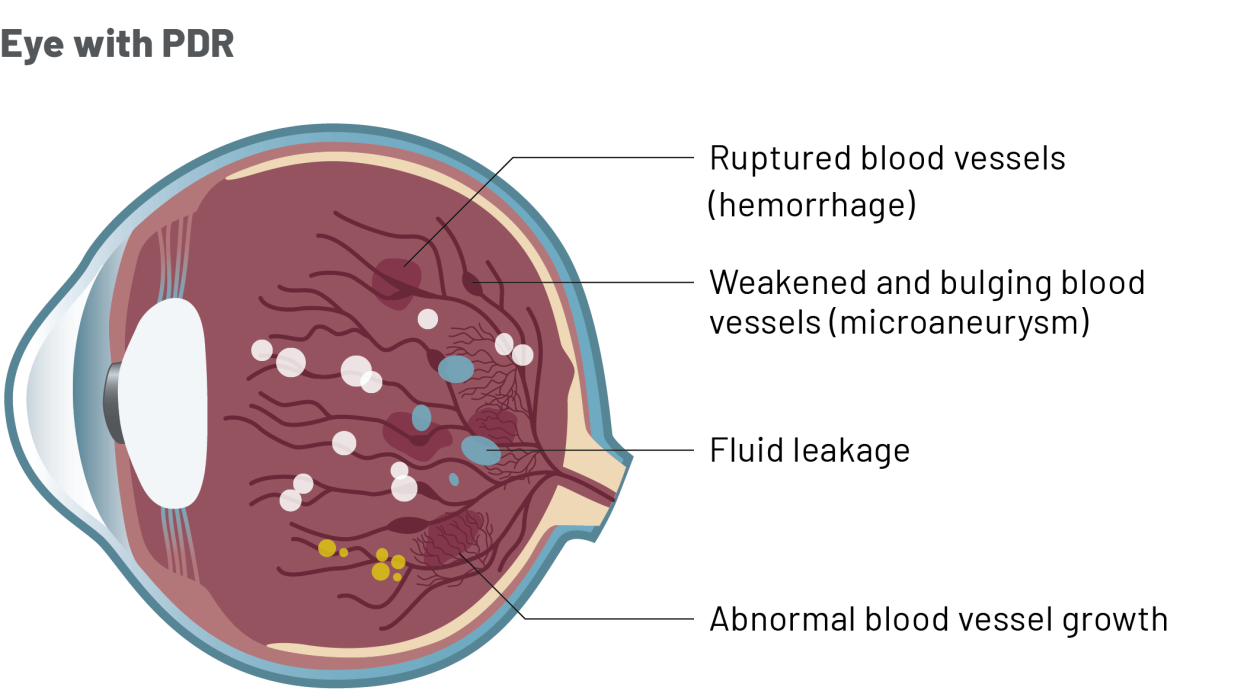
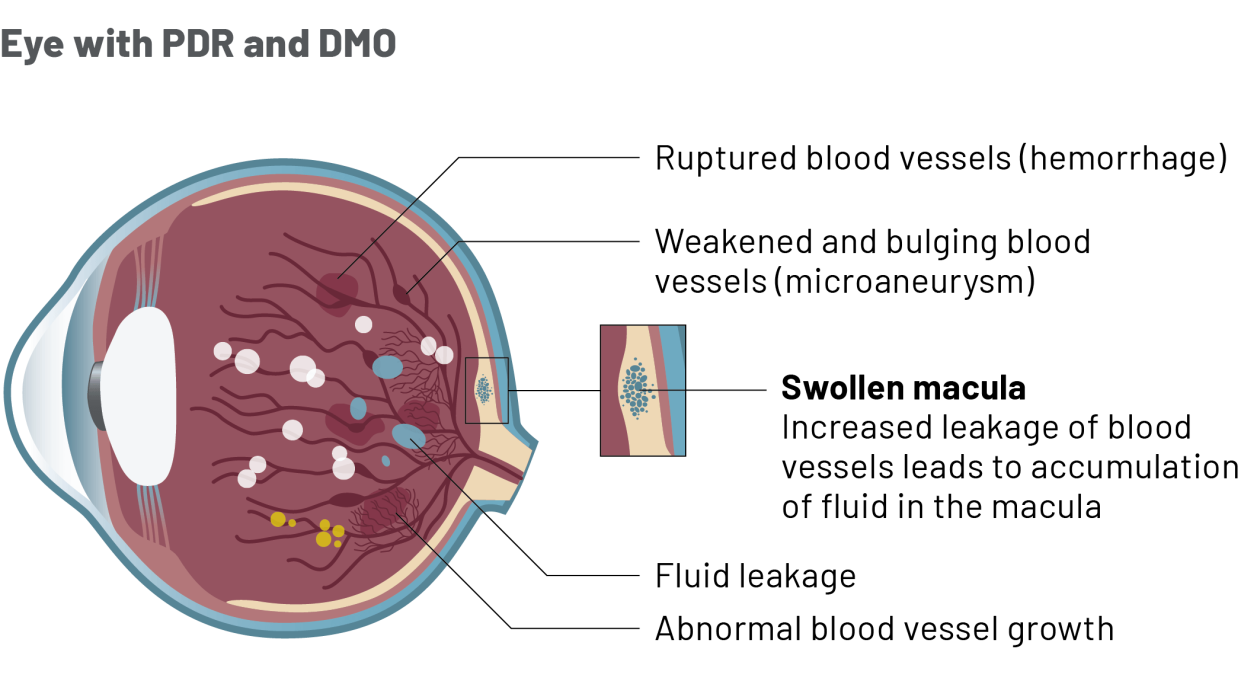
How is Ongavia treatment given?
Ongavia is given by your ophthalmologist (eye doctor) as an injection into the eye.
It is normal to worry about such injections, but usually the injection is virtually painless.
What will happen at my treatment appointment?
On the day of your treatment, care will be taken to make sure you are relaxed and comfortable.
Before receiving Ongavia, it is important to tell your doctor if:
- You have had a stroke or experienced transient signs of stroke (weakness or paralysis of limbs or face, difficulty speaking or understanding).
- You are taking or have recently taken any other medicines, including medicines obtained without prescription.
- You have an eye infection.
- You have any pain or redness in your eye.
- You think you may be allergic to Ongavia, to other medicines with ranibizumab or to Betadine® (iodine).
Keeping your doctor informed will help them to decide whether Ongavia is the most appropriate treatment for you.
Prior to the treatment a doctor or nurse will:
- Cover your face and the area around the eye with a special drape.
- Clean your eye and the skin around it.
- Hold your eye open so you do not blink.
- Numb your eye with an anesthetic to prevent pain.
The doctor will then give the injection into the white part of your eye. You may feel a little pressure with the injection.
What will happen after I receive my Ongavia injection?
Your doctor will perform eye tests, such as measuring the pressure in your eye, to make sure the treatment went well.
The white area of the eye, where the injection is given, will likely be red.
- This redness is normal and it will go away in a few days.
You may see a few spots or ‘floaters’ in your vision.
- These spots are normal and should go away in a few days.
Contact your doctor if either of these symptoms do not go away or get worse.
Your pupils will be dilated for the injection, and this can make it difficult for you to see for a few hours after the treatment.
- You should not drive until your vision has returned to normal.
It is important to monitor any changes in the condition of your eye and your overall well-being in the week following your injection.
Rarely, injections in the eye can cause infection.
What to do if I have side effects?
Contact your doctor as soon as possible if you have any of the following signs and symptoms in your eye:
- Pain
- Light sensitivity/ tearing
- Swollen lids or other swelling
- Increasing redness
- Blurred, distorted or sudden loss of vision
- Light flashes
- Seeing flies, black spots or coloured halos
- Drying of the surface of your eye.
If you notice any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in the package leaflet.
You can also report side effects directly via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard
Please ensure that you provide the brand name (or product licence number and manufacturer), and the specific batch-number.
By reporting side effects you can help provide more information on the safety of this medicine.
How long will I need to continue Ongavia treatment?
Every patient is different. It is likely that you will need additional Ongavia or other ranibizumab injections, but this will depend on how you respond to treatment and how your vision changes.
If you are considering stopping treatment with Ongavia, ask your doctor for advice first.
For any further questions on the use of this product, please ask your doctor.
Follow all your doctor’s advice carefully. They may differ from the general information in this guide.
Your doctor will decide how often they wish to see you to monitor your condition and determine if you need additional injections.
Always go to every appointment that your doctor arranges for you.
If you miss an appointment for Ongavia treatment, contact your doctor as soon as possible.
If you have any further questions, ask your doctor or pharmacist.
If you experience any signs or symptoms that you consider to be associated with the use of Ongavia, but are not listed in this guide, please tell your doctor.
This guide is provided for your education and complements the Patient Information Leaflet.